
- Prof. Dr. Jürgen Gauß
- In quantum chemistry, the electronic structure of molecules is investigated based on a quantum mechanical description. In addition, important molecular properties such as energy, structure, etc. are calculated.
For chemically relevant molecular systems consisting of a large number of electrons (up to several hundreds of atoms and several thousands of electrons) the relevant equations, i.e., the electronic Schrödinger equation, cannot be solved in closed form. Therefore, one key aspect of quantum chemistry is the develpoment of numerical approximations for the solution of these equations.
Important topics that are under study at present include
- development of methods to describe electron-correlation effects
- design of methods using analytic energy derivatives for the computation of pro-perties
- implementation of techniques of response theory for the study of electronic exci-tations
- development of methods to treat relativistic effects on molecular properties
Apart from these methodological studies the application of quantum-chemical methods to the investigation of chemically relevant problems constitutes the important field of computational chemistry. Examples for such applications are shown below:
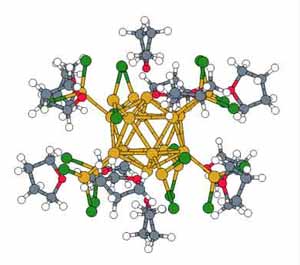
Calculated structure of a complex aluminum-chloride cluster consisting of 198 atoms and 1106 electrons.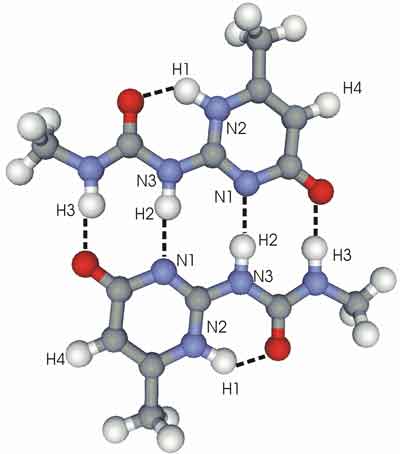
Calculated atomic distances in molecules exhibiting hydrogen bonds. The indicated distances vary from 0.1 to 0.3 nanometers.


