By suitably combining two or more organic molecules, specific crystal structures can be formed which lead to the formation of conjugated charge systems. As a result, an electrical conductivity comparable with metals can be achieved. By selectively varying the chemical substituents, there are many possibilities to modify the electronic properties and to synthesize new compounds. Even slight changes can have a very great influence on the physical behavior of organic molecules.
An example of such a molecular system are charge transfer salts. These are composed of two different molecules, between which a charge exchange takes place. Precondition for the formation of such a complex is a low ionization energy of the one molecule (donor), as well as a high electron affinity of the other molecule (acceptor), so that an electron transfer between the molecules can take place.
The combination of donor and acceptor results in the partial depopulation of fully occupied electron states, which can lead to electronic conductivity.
In this project, the charge transfer salt TMP-TCNQ consisting of tetramethoxypyrene (TMP) and 7,7,8,8-tetracyanoquinodimethane (TCNQ) is examined by means of scanning tunneling microscopy and spectroscopy. By measuring differential tunneling spectra, the relative positions of the HOMO and LUMO states are measured relative to the Fermi energy of the metal substrate, which are critical for electron transport through the metal-organic interface. Figure 1 shows an STM image of a monolayer TCNQ on the Au (111) surface.
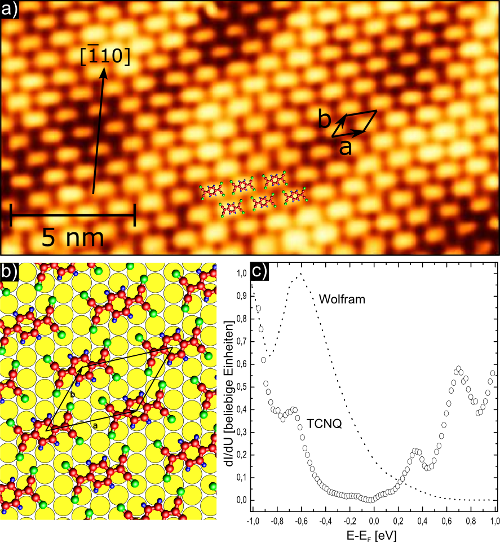
Figure 1:
a) STM image of TCNQ on Au (110).
b) Model of the adsorption sites of the TCNQ molecules on the Au (111) surface.
c) Differential tunneling spectrum from TCNQ to W (110).
A further interesting example of organic charge transfer salts is k-(BEDT-TTF)2Cu[N(CN)2]Br. This complex shows superconductivity below a critical temperature. By means of tunneling spectroscopy, an energy gap at the Fermi energy can be observed on a k-(BEDT-TTF)2Cu[N(CN)2]Br crystal at temperatures up to 13.32 K (see Fig. 2).

Figure 2: Differential cell conductivity of k- (BEDT-TTF) 2Cu [N (CN) 2] Br measured at different temperatures.
In this research, electronic correlation effects in organic charge transfer salts are investigated using scanning tunneling microscopy and scanning tunneling spectroscopy. The temperature dependence of the single-particle state density near the superconducting phase transition for k- (BEDT-TTF) 2Cu [N (CN) 2] Br and its deuterated variant is measured in a constant magnetic field.