
Benjamin Weiss
Biomedical Scientist in Histology and Cytopathology
Evolutionary Ecology
Institute of Organismic and Molecular Evolution
Hanns-Dieter-Hüsch-Weg 15
55128 Mainz, Germany
Phone (office): +49 - (0) 6131 - 39 24424
Phone (lab): +49 - (0) 6131 - 39 23944
Fax: +49 - (0) 6131 - 39 23731
Methods and Research interests
Interactions between insects and microbial symbionts are quite common in nature. These symbioses are not only very diverse in their function, but can also show great differences in their morphology and localization.
A fundamental part of our research is to localize these symbioses, characterize them by molecular methods and understand their functions. Symbionts can be located in different ways at very different places in the insect body. They are for example on the insect surface, in invaginations, freely in the intestine or in special organs, so-called bacteriomes. Histology is an important method for the localization and description of these structures, which also enables us to detect rRNA-specific microorganisms combining with molecular methods. For this purpose, the insects are embedded in wax or plastic, from which sections with a thickness of a few micrometers can be made. These thin sections serve as the basis for various microscopic methods and allow us to assess the position, morphology and histochemical properties of these structures, as well as rRNA-based detection of bacteria and fungi by fluorescence in situ hybridization (FISH).
The method spectrum of our histological laboratory includes embedding in paraffin, methyl and butyl methacrylates, hydroxyethyl methacrylates (Technovit 8100, LR white) and epoxy resins. In addition to the classical overview staining methods such as hematoxylin-eosin (HE), AZAN, Casson, toluidine blue-pyronine, we also offer histochemical detection methods such as Millon, PAS, Feulgen, alcian blue-nucleus red; microbiological staining methods such as Gram, Grocott, Warthin-Starry, fluorescence in situ hybridization (FISH) and hybridization chain reaction (HCR). Furthermore, it is also possible to perform whole-mount in situ hybridizations on whole insect organs or larvae or to create 3D reconstructions from histological section series.
 |
 |
 |
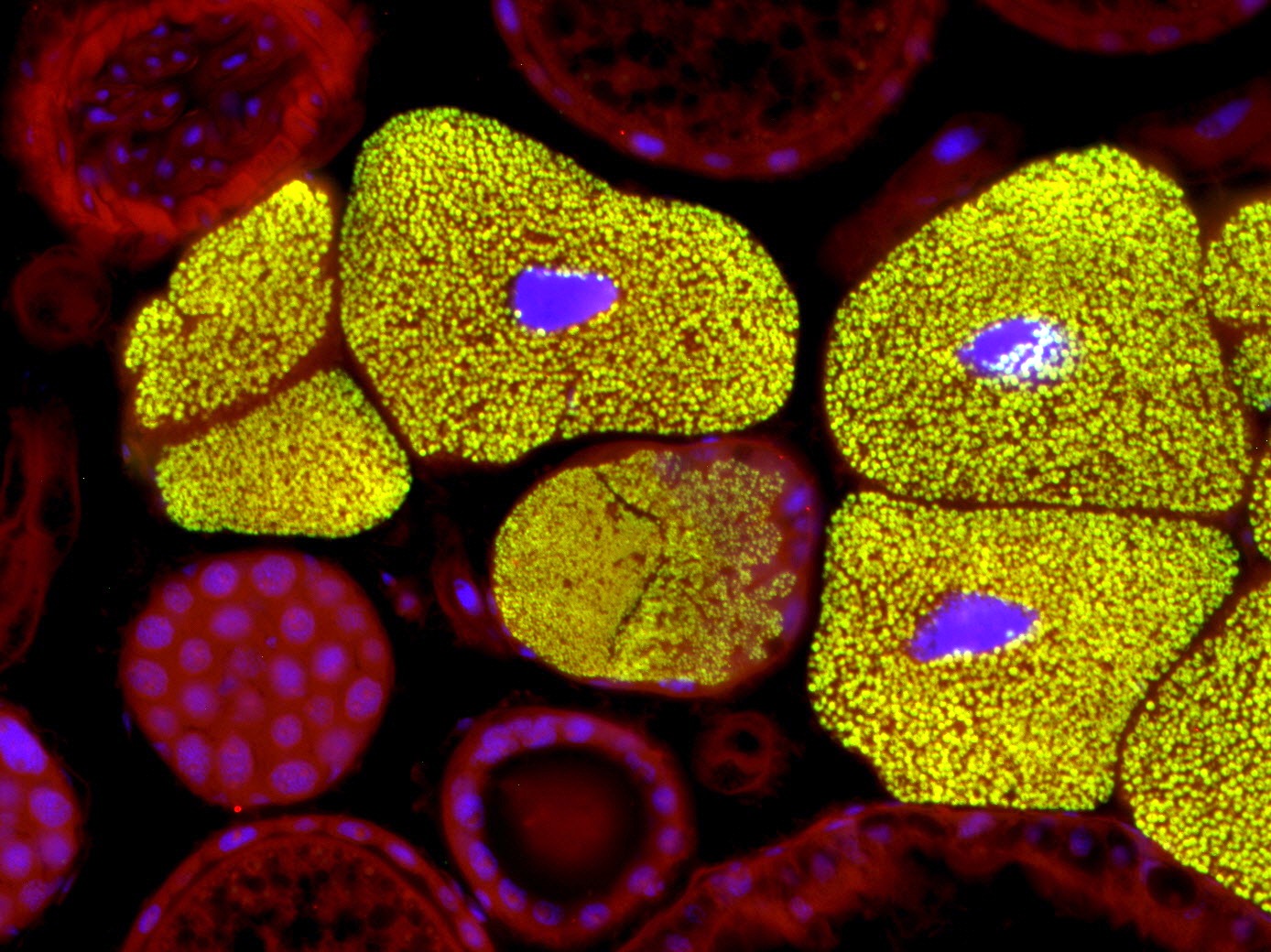
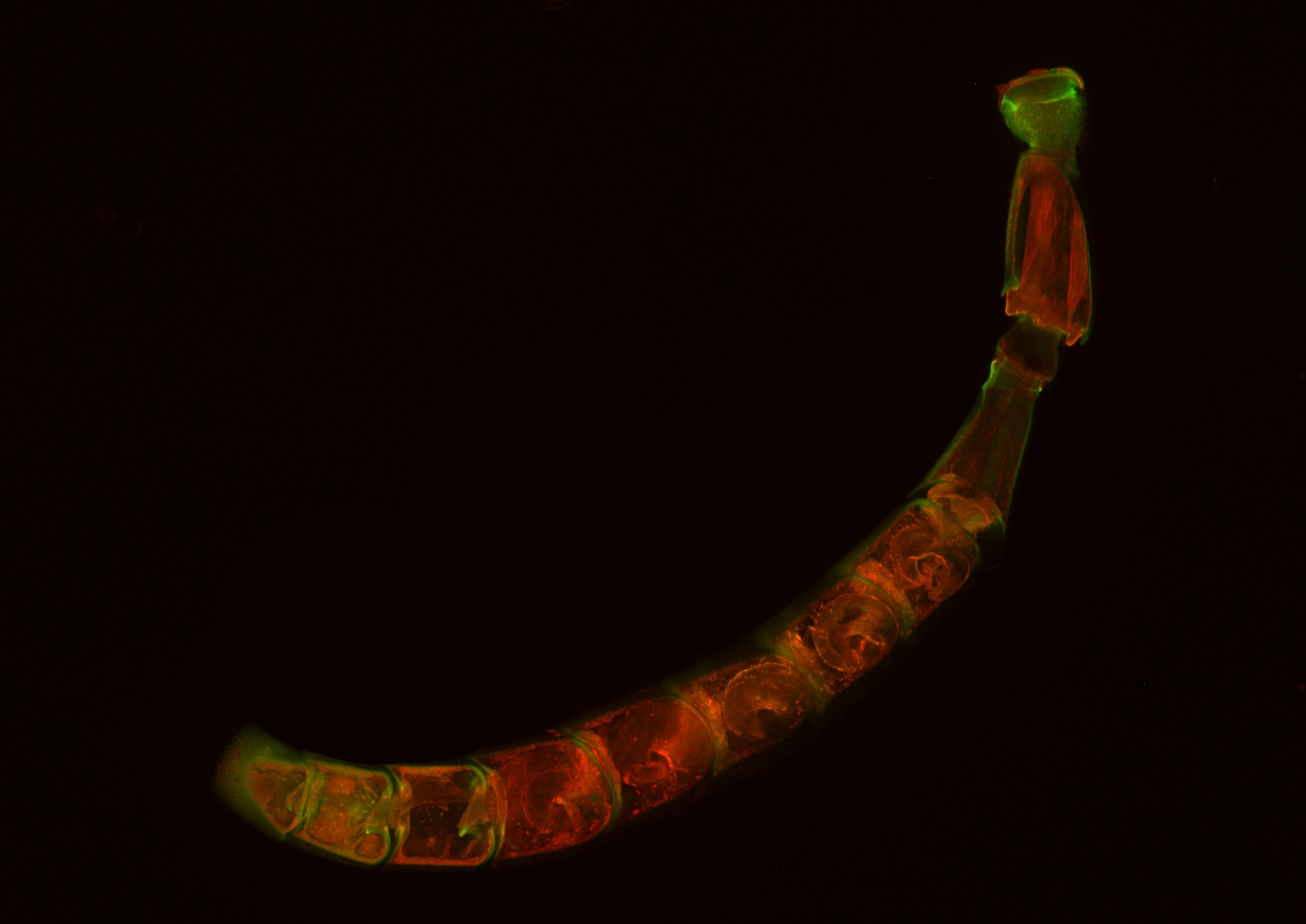
| Bacteriomes of Dasytes virens. Fluorescence in situ hybridization on semi-thin section of Technovit 8100 (5 µm). DAPI (blue), specific bacterial probe (green), autofluorescence (red). | Bee wolf antenna, whole mount fluorescence. The antenna was embedded in butyl methacrylate, sectioned sagittally and released from the embedding medium with xylene. Autofluorescence (excitation with 581and 675 nm). |
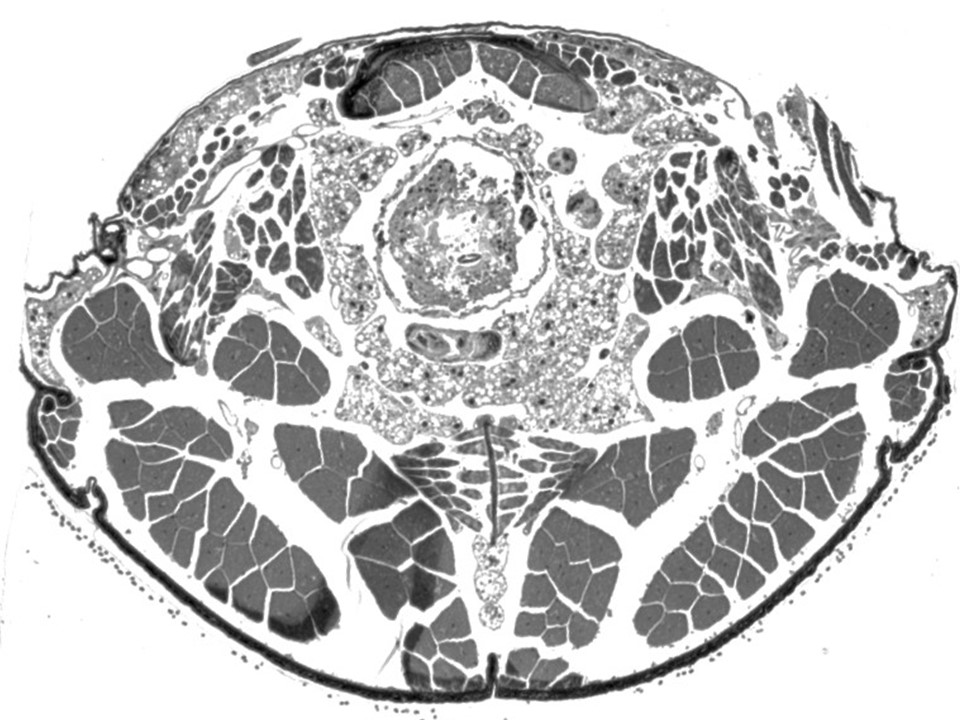
Transverse section through the thorax of Dasytes virens. Embedded in epoxy resin, section thickness 1 µm, toluidine blue-pyronine staining. |
