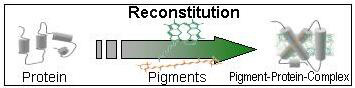
LHCII is the major light-harvesting complex of photosystem II in the photosynthetic apparatus of higher plants. Its apoprotein is the most abundant membrane protein in plants and binds 50 % of their chlorophyll. Not only does LHCII harvest light energy for photosynthesis but it is also involved in the dissipation of excess excitation energy, in balancing the energy flow between photosystems I and II, and in thylakoid stacking in grana structures. LHCII is one of the few membrane proteins whose structure is known and that can be re-folded in vitro. This opens up numerous experimental options for understanding the functions of this important membrane protein in relation to its structure.
We take advantage of the possibility to reconstitute bacterially expressed LHCII apoprotein with pigments in vitro to yield a structurally authentic pigment-protein complex. With spin labels introduced into the complex, LHCII becomes amenable to electron-paramagnetic resonance (EPR) spectroscopy. This allows to assess the structure of individual protein domains as well as their structural dynamics (collaboration with Prof. Gunnar Jeschke, ETH Zurich; Ref.: Jeschke et al., 2005; Volkov et al., 2008; Dockter et al., 2009; Dockter et al, 2012; Fehr et al., 2015, 2016). EPR and other spectroscopic techniques (fluorescence, circular dichroism – CD) are employed to detect possible conformational changes of LHCII in its various functional states. Moreover, we are interested in how the properties of membrane-inserted LHCII depend on the lipid composition of the membrane (Ref.: Yang et al., 2006) and how it behaves in artificial membranes (Ref.:Zapf et al., 2015).