Synthesis and application of triphenylene ketale based supramolecular receptors
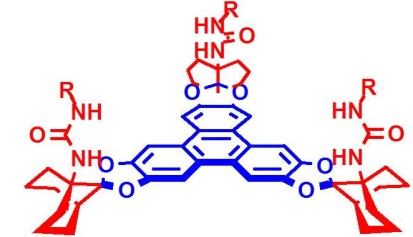 An unusual large and rigid backbone providing concave and convergent preorganized functional groups for supramolecular interaction was established by us recently. Based on these functionalized triphenylene ketals, the first artificial caffeine receptors were developed (see right), and chirally modified receptors proved to be capable of the first enantiofacial discrimination of single heterocyclic guest molecules.
An unusual large and rigid backbone providing concave and convergent preorganized functional groups for supramolecular interaction was established by us recently. Based on these functionalized triphenylene ketals, the first artificial caffeine receptors were developed (see right), and chirally modified receptors proved to be capable of the first enantiofacial discrimination of single heterocyclic guest molecules.
Triphenylene ketals are novel C3-symmetric scaffolds with nano-scale dimensions. Groups of appropriate affinity in well defined orientations are the key for high selectivity and association constants in these artificial receptors. Due to their rigidly oriented and conformationally locked functional groups, these systems are therefore among the most potent systems for molecular recognition.
Catechol ketals of isosteviol offer an access to a rigid chiral cleft (see left). The starting material is easily accessible in large scale from the natural product stevioside.
For the trimerization process of the construction of the backbones we developed several methods based on oxidative coupling in chemical and electrochemical manners.
Lit:
M. Bomkamp, K. Gottfried, O. Kataeva, S. R. Waldvogel, Synthesis 2008, 1443.
M. C. Schopohl, A. Faust, D. Mirk, R. Fröhlich, O. Kataeva, S. R. Waldvogel, Eur. J. Org. Chem. 2005, 2987.
M. C. Schopohl, C. Siering, O. Kataeva, S. R. Waldvogel, Angew. Chem. Int. Ed. 2003, 42, 2620.
